【General】Oil Repellency test -(Principle and experimental test results)
2022-12-30
Ⅰ、 Principle of material surface wetting
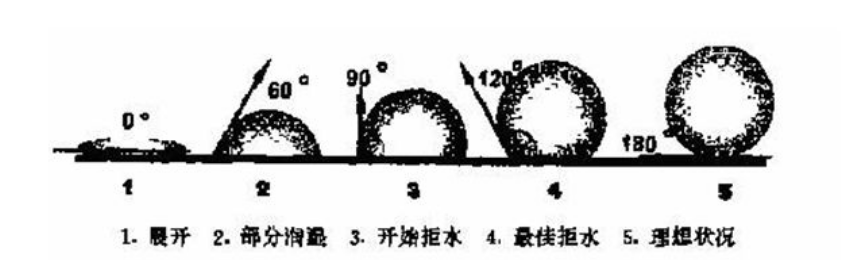

Membrane material in the so-called wettability, this is such as water, ink, adhesives, coatings to plastic film, paper or metal foil adhesion basis. Wettability determines the ductility of a liquid over a solid surface. For example, water spreads out into a thin layer on a hydrophilic surface, but forms droplets on an oleophobic surface. The contact Angle formed between the tangents of the outer surface of the water drop and the solid surface (Figure 1, Angle θ), that is the wettability of the surface. The larger the contact Angle is, the worse the wettability is. In the physical , the strict thermodynamic definition of wetting is: after contact between a solid and a liquid, the enthalpy of the system (solid and liquid) decreases, which is called wetting.
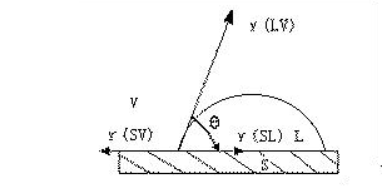

Figure 1 Schematic diagram of wetting tension on solid-liquid phase
As shown in the figure above, the physical model is examined. From the thermodynamic point of view, when the droplet falls on a clean and smooth solid surface, ignoring the effects of gravity and viscosity of the liquid, the droplet spread on the solid surface is determined by three interfacial tensions: solid-gas (SV), solid-liquid (SL) and liquid-gas (LV). The equilibrium relationship is determined as follows
γSV=γSL+γLV cosθ
F=γLV cosθ=γSV-γSL+γLV
Where, θ is the wetting Angle, F is the wetting tension, and γ is the interfacial energy. Obviously, when θ > 90°, the wetting tension is small and does not moisten. θ < 90° is wet; When θ=0°, the wetting tension F reaches its maximum and can be fully wetted, that is, the liquid spreads freely on the solid surface. (The main cause of interfacial energy γ is the imbalance of van der Waals forces and chemical bond forces at the interface. These forces cancel each other out because they are symmetrical inside the solid, and they cannot be symmetrical at the interface, thus showing the interface force, namely the interface energy). And you can see that the prerequisite for what we call wetting is gamma SV > gamma SL, or gamma SL is very small. So if you want to achieve the effect of oil drainage, it means that the larger the contact Angle, the better, the smaller the tension;
Ⅱ、Surface Tension of Common Plastics (Documentation)
Table 1 Surface tension of plastics(dyn/cm, 25℃)
|
Plastic |
Surface tension |
Plastic |
Surface tension |
|
PTFE |
18.5 |
PVC |
39.0 |
|
PVF |
28.0 |
PMMA |
39.0 |
|
PCTFE |
31.0 |
PVDC |
40.0 |
|
PE |
31.0 |
PET |
44.0 |
|
PS |
33.0 |
PP |
29 |
So PTFE is a plastic with very low surface tension.
Ⅲ、Surface Tension of Common Liquids (Documentation)
Table 2. Surface tension of several typical liquids(dyn/cm,25℃)
|
Liquid |
Surface tension |
Liquid |
Surface tension |
|
Water |
72.88 |
methyl alcohol |
22.6 |
|
caster oil |
36 |
ethylene glycol |
30 |
|
29 |
30 |
||
|
24 |
Lithium hexafluorophosphate |
36 |
|
|
24 |
59.13 |
||
|
78.97 |
29.6 |
||
|
IPA |
22 |
\ |
\ |
Ⅳ、Test method for Oil Repellency (AATCC118-2002 Oil Repellency: Impedance Test of Hydrocarbons)
1.The oleophobic test was performed by the American Association of Textile Chemists and Printmakers (AATCC) Standard 118-1989.
1.1The test method is to drop a liquid with known surface tension on the surface of the material, observe the wetting condition, adsorption and contact Angle, and evaluate the oil repellent property of the material. The oil repellent grade is the highest number of the non-wetting test liquid.
2. Test devices and reagents
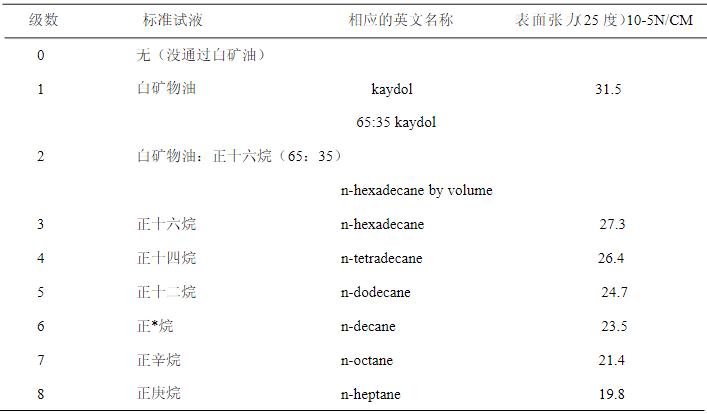
2.1 Prepare the reagents according to the table as below.
2.2 Drop bottle is usually use 30ml with a label of the name of the reagents on the outside
2.3 Rubber suction
2.4 White AATCC absorbent paper
2.5 Gloves
2.6 Standard test reagents

3.Test Sample
3.1 Two specimen size 20x20cm
3.2 Samples are placed in standard atmosphere for at least 4 hours before testing
3.3 Test procedure
3.3.1 Place the sample flat on a smooth surface and pad white absorbent paper underneath the sample. If the sample is relatively thin, two layers of sample should be used to avoid wetting the bottom surface with the test solution, so as to ensure that the grade is not affected by the wetting of the bottom lining paper.
3.3.2Drop the reagent from the lowest grade of 1 at 5 locations along the weft direction of the test sample (the diameter of the oil drops is approximately 5mm or the volume is 0.05ml, and the reagent drops are at least 4.0cm apart). Drop the reagent about 0.6cm away from the sample
3.3.3Observe the wetting condition of the droplet within 30±2s from an Angle of about 45°. If there is no wetting phenomenon, drop a drop of higher rate test reagent.
3.3.4 Repeat the above procedure until obvious wetting appears under or around the droplet within 30±2s. Normal signs of sample wetting are darkening of the sample around the droplet, disappearance of the droplet, exocurination of the droplet, or disappearance of the gleam of the droplet
3.4.5 If 3 or more drops of the drop pass, this level passes. Conversely, a failure of 3 or more drops indicates a failure
4.Test report
The test report should contain the following:
4.1 If 2 samples have the same oil repellency grade, report this grade
4.2 If the grade of the two samples is different, the third sample will be tested again. If the result of the third sample is the same as that of any of the first two samples, the grade of the third sample is reported as the same
4.3 If the grade of the third sample is different from the first 2 samples, report the intermediate grade with the oil repellency number accurate to 0.5 grade
Ⅴ、PTFE waterproof and breathable membrane Oleophobic test by SST
1.Prepare 7 test liquids (water, oil, formamide, methanol, hydrogen peroxide, ethylene glycol ether, alcohol) with their names and surface tensions as follows:
|
NO. |
Liquid |
Surface tension dyn/cm |
|
1# |
Water |
72.88 |
|
2# |
Oil |
36 |
|
3# |
formamide |
59.13 |
|
4# |
methanol |
22.6 |
|
5# |
hydrogen peroxide |
78.97 |
|
6# |
ethylene glycol ether |
30 |
|
7# |
alcohol |
24 |
2.Choose two type of test sample (①BD06200300-ASY oleophobic ②BD06200300 non-oleophobic )L*W=10*5CM
3.Follow the <AATCC118-2002 oil repellent>test procedure
- 1)Place the sample on a smooth surface with white absorbent paper underneath the sample
- 2)Drop the reagent vertically along the direction of the test sample from above (the diameter of the reagent is about 5mm, and the reagent is at least 0cm apart). Drop the reagent about 0.6cm away from the sample.
- 3)Observe the wetting of the droplet within 30±2s from an Angle of about 45°. If no wetting occurs, drop another test solution
- 4)Repeat the above procedure until the sample is visibly wet under or around the droplet within 30±2s. Normal signs of sample wetting are darkening of the color around the sample, disappearance of the reagent, exocircuation at the reagent, or disappearance of the reagent flash
- 5)If 3 or more drops of the reagent pass, the sample passes the test. Conversely, failing three drops or more means failing the test



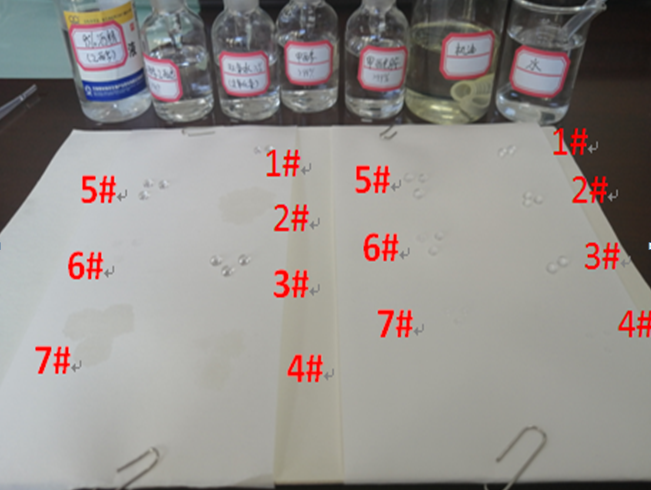


4.Test result
①It can be seen from the figure that the non-oleophobic product BD06200300, reagent 2# oil (36 dyn/cm), 4# methanol (22.6 dyn/cm), 7# ethanol (24 dyn/cm), no oil rejection ability, the lower the surface tension of the liquid, the more obvious the wetting effect;
②It can be seen from the figure that BD06200300-ASY has an oleophobic effect on all test reagents, but the lower the surface tension of the liquid, the smaller the contact Angle θ, the larger the wetting tension, the more likely to wet the material.
③The waterproof and air permeable membrane BD06200300-ASY of can reject methanol (22.6dyn/cm), achieving the 7 oil repellent effect of AATCC118 standard.

